DynaMut analysis and prediction of protein stability changes upon mutation using Normal Mode Analysis
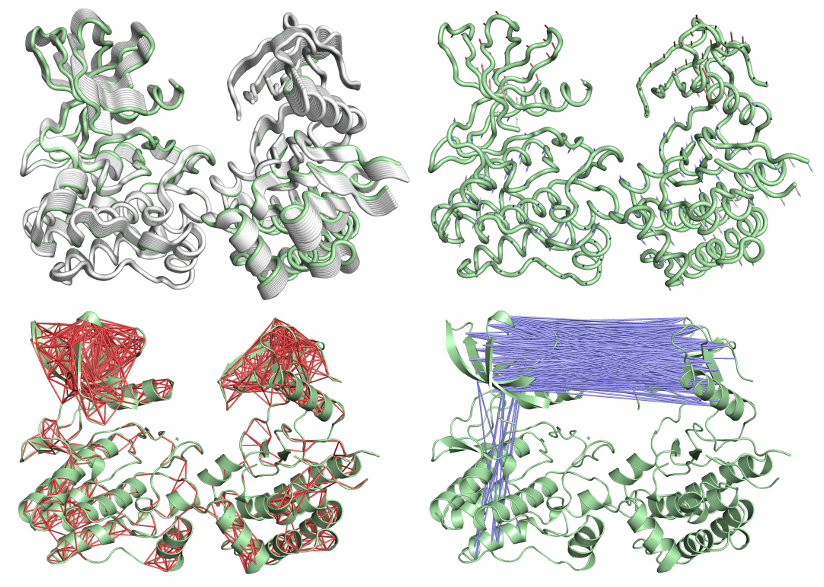
Abstract: Proteins are highly dynamic molecules, whose function is intrinsically linked to their molecular motions. Despite the pivotal role of protein dynamics, their computational simulation cost has led to most structure-based approaches for assessing the impact of mutations on protein structure and function relying upon static structures. Here we present DynaMut, a web server implementing two distinct, well established normal mode approaches, which can be used to analyse and visualise protein dynamics by sampling conformations and assess the impact of mutations on protein dynamics and stability resulting from vibrational entropy changes. DynaMut integrates our graph-based signatures along with normal mode dynamics to generate a consensus prediction of the impact of a mutation on protein stability. We demonstrate our approach outperforms alternative approaches to predict the effects of mutations on protein stability and flexibility (p-value < 0.001), achieving a correlation of up to 0.70 on blind tests. DynaMut also provides a comprehensive suite for protein motion and flexibility analysis and visualisation via a freely available, user friendly web server.
Available Resources
|
|
Normal Mode Analysis of 3D structures |
| Mutation effects prediction | Prediction of protein stability change upon mutation |